Those ages 12 through 15 can still receive the vaccine under the existing emergency use authorization. -- Pfizers COVID-19 vaccine was granted an emergency use authorization for 12- to 15-year-olds by the US.
Mhra Authorises Pfizer Biontech S Covid 19 Vaccine For 12 To 15 Year Olds Pharmatimes
Pfizer vaccines for kids age 12-15 could be administered as soon as Thursday Woodcock said so long as all goes well at Wednesdays Advisory Committee on.

Pfizer fda approval 12 15. No Covid vaccines have been authorized or approved. In participants aged 12-15 years old BNT162b2 demonstrated 100 efficacy and robust antibody responses exceeding those reported in trial of vaccinated 16-25 year old participants in an earlier analysis and was well tolerated The companies plan to submit these data to the US. Normally doctors can prescribe FDA-approved products.
The Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by the US. The PfizerBioNTech vaccine has been authorized for. Verywell Nez Riaz.
The FDA has authorized but not yet fully approved the vaccine for adolescents ages 12 to 15. This announcement of Emergency Use Authorization EUA was made on Monday May 10 and signals a major step forward in the national effort to get every citizen inoculated. May 3 Reuters - The US.
The FDAs full approval applies to people 16 and older. Food and Drug Administration FDA and the European Medicines Agency EMA as soon as possible to. The Food and Drug Administration FDA has approved the Pfizer-BioNtech COVID-19 vaccine for use in children aged 12 to 15 years of age.
The vaccine also continues to be available under emergency use authorization EUA including for individuals 12 through 15 years of age and for the administration of a third dose in certain. FDA authorizes Pfizer vaccine for children 12 to 15 The CDC is expected to sign off on the move Wednesday making Thursday the first day shots could be available for children 12 to 15 years old. Food and Drug Administration is preparing to authorize Pfizer Inc PFEN and German partner BioNTech SEs 22UAyDE COVID-19 vaccine for adolescents aged between 12 and.
The FDA approval for Pfizers vaccine covers everyone aged 16 and above. Food Drug Administration on Monday. Fauci on Pfizer vaccines FDA approval approving Moderna and Johnson Johnson shots Aug 23 2021 655 PM EDT.
Pfizer said that once six months of full data was available on that age group it would also submit a new application for full approval for 12- to 15-year-olds to receive the drug. This is the first coronavirus vaccine approved by the FDA and is expected to open the door to more vaccine mandates. FDA approves Pfizer vaccine for 12-15-year-olds DURHAM NC.
Pfizers shot is still being dispensed to 12- to 15-year-olds on an emergency basis. The mRNA-based vaccine has been available since late last year through an Emergency Use Authorization EUA and will continue to be offered under that designation for those aged 12 to 15 until. 104 Zeilen The FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include 12 15 year olds and issued an updated FDA COVID-19 Response At-A-Glance Summary.
While Mondays approval doesnt cover young people ages 12 to 15 they can still get the Pfizer vaccine through emergency use authorization. Max Cuevas 12 holds his mothers hand as he receives the Pfizer COVID-19 vaccine from nurse practitioner Nicole Noche at Families Together of Orange County in Tustin Calif May 13 2021. Food and Drug Administration FDA but has been authorized for emergency use by FDA under an Emergency Use Authorization EUA to prevent Coronavirus Disease 2019 COVID-19 caused by severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 for use in individuals 12 years of age and older.

Fda Set To Authorize Pfizer Covid 19 Vaccine For Children Ages 12 To 15

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15

Fda Oks Pfizer Covid 19 Vaccine For 12 15 Age Group Coronavirus Updates Npr

Pfizer Seeks Full Fda Approval For Covid 19 Vaccine Coronavirus Updates Npr
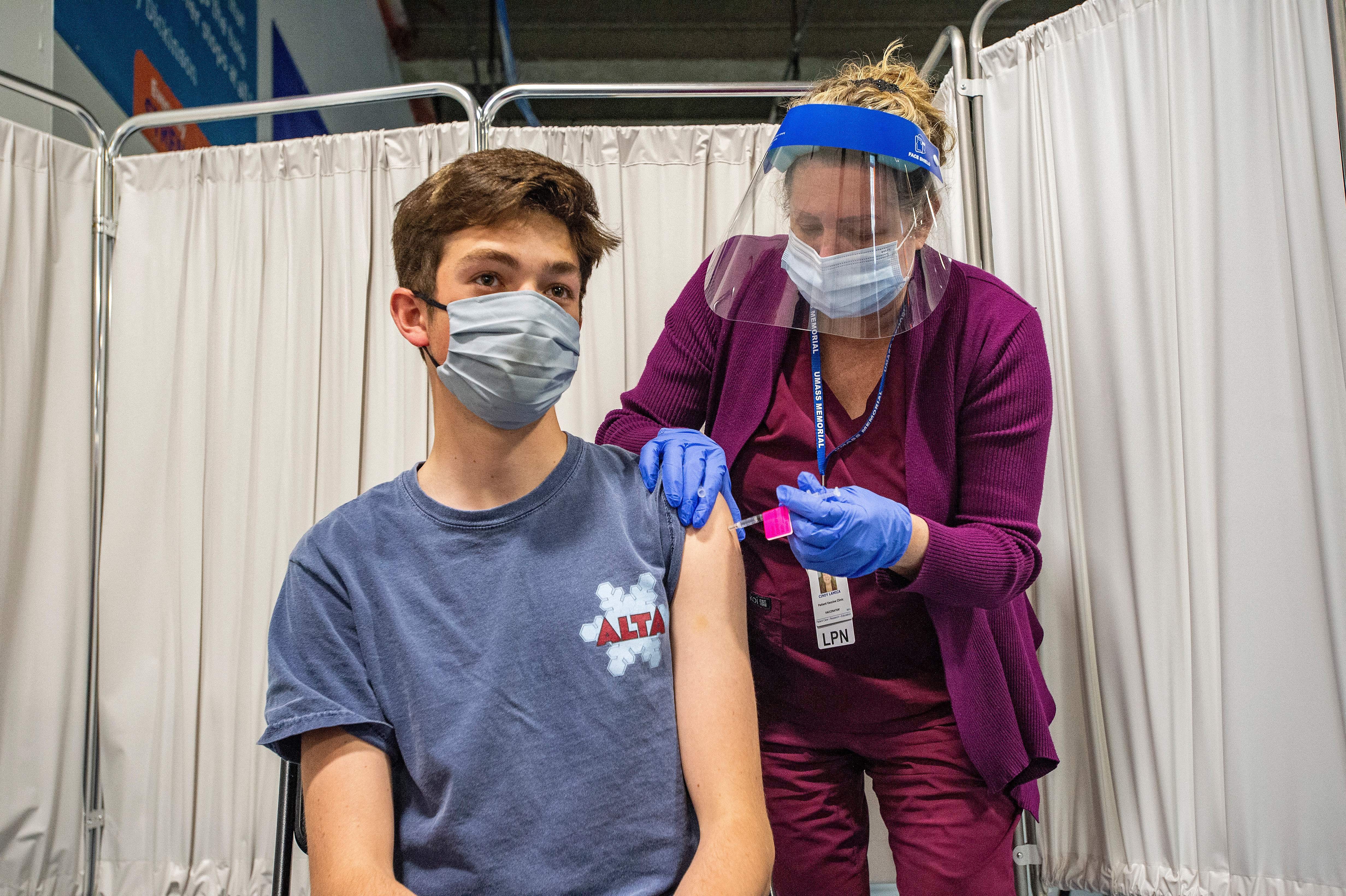
R I Parents Can Now Begin Registering Kids Age 12 15 For Pfizer Vaccine The Boston Globe
.png)
Cdc Approves Pfizer Covid 19 Vaccine For 12 15 Year Olds

Fda Appears Poised To Authorize Pfizer Biontech Vaccine For Adolescents By Next Week The Washington Post
Fda Staff Recommends Watching For Bell S Palsy In Moderna And Pfizer Vaccine Recipients

Pfizer Says Fda Will Soon Authorize Covid 19 Vaccine For 12 15 Age Group Coronavirus Updates Npr
Israel May Begin Vaccinating 12 To 15 Year Olds By End Of May The Times Of Israel
Pfizer Vaccine Approved For Ages 12 15 What About Ages 6 11 Cbs8 Com

Covid Vaccine Moderna Applies For Full Fda Approval

Pfizer S Covid Vaccine Gets Green Light For Children As Young As 12 Fortune
Pfizer Vaccine Approved For Children Ages 12 15 Cnet
Covid Vaccine Fda May Authorize Pfizer S This Week

Fda Authorizes Pfizer Covid 19 Vaccine In Adolescents 12 And Up

Teenagers Aged 12 15 Can Now Receive The Pfizer Vaccine Department Of Public Health City Of Philadelphia
/cdn.vox-cdn.com/uploads/chorus_image/image/69763297/1234462203.0.jpg)

